|
Getting your Trinity Audio player ready…
|
Today I am sharing how I made a superconductor in college.
It was for a class – senior lab – and I ended up having way too much fun as always.
A superconductor is a substance capable of being superconducting at low temperatures.
By low temperatures, I mean temperatures below 30 Kelvin.
For my American readers, 30 Kelvin is -405.67 Fahrenheit.
For everyone else, 30 Kelvin is -243.15 Celcius.
Superconductors have many applications such as to make the fastest trains in the world!
However, it is challenging to maintain super low temperatures for practical applications.
Some special superconductors are able to superconduct at relatively high temperatures!
These high-temperature superconductors are incredibly useful and a hot topic of research.
YBCO is one of them.
It becomes superconducting at around 92 Kelvin.
That is above the boiling point temperature of liquid nitrogen, the stuff you make liquid nitrogen ice cream with!
Liquid nitrogen boils at 77 Kelvin.
So getting to the superconducting or critical temperature of YBCO requires help only from our good friend, liquid nitrogen.
Easy peasy.
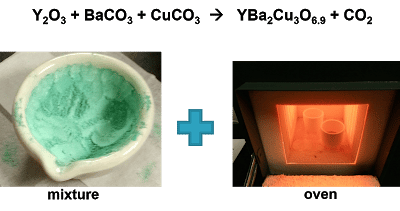
Full form of YBCO: Yttrium Barium Copper Oxide
Just a chemical compound that can be made from the proper ingredients as shown in the picture above!
I love chemistry so I went ahead and made my own YBCO in senior lab, teaming up with my friend, James.
To be considered a superconductor, a material must display two distinct properties:
zero resistance + perfect diamagnetism
At the critical temperature, the material undergoes a phase transition and enters its superconducting phase.
Just like water boils at 100 degree Celcius and is considered to be changing phase from liquid water to water vapor, YBCO starts to undergo a phase transition at 92 Kelvin (about -182 degree Celcius) and enters its superconducting phase.
You are probably familiar with electric current and electric resistance.
But, what is diamagnetism?
A perfect diamagnet is a material that allows zero magnetic induction in its interior when an external magnet is brought near it.
So, basically, a perfect diamagnet repels an external magnet.
So, when we brought a small magnet near our superconducting sample of YBCO, it repelled this magnet and made it levitate just above its surface.
Aaaaaaaaaaa, so cool!
The two main aspects of making YBCO *at home* are:
mixing + baking
It was like baking a cake!
We first made sure to mix up all our ingredient chemicals in the correct proportions and then programmed our oven to heat and cool appropriately so as to crystallize our mixture and give us YBCO pellets.
And then we used liquid nitrogen to cool the YBCO pellet down to its critical temperature.


Leave a Reply